Fukui Kenichi was born the eldest of three sons of Ryokichi Fukui, a foreign trade merchant and factory manager, and Chie Fukui, in Nara, Japan, on October 4, 1918. In his high school years, chemistry was not his favourite subject, but the most decisive occurrence in his educational career came when his father asked the advice of Professor Gen-itsu Kita of Kyoto Imperial University concerning the course he should take.

Prof. Kita suggested that Ryokichi, one of his juniors from the same native province, should send Fukui Kenichi to the Department of Industrial Chemistry with which he was then affiliated.
His first scientific delight came in 1952 when he found a correlation between the frontier electron density and the chemical reactivity in aromatic hydrocarbons. This success led his theoretical group to the chemical reactivity theory, extending more and more widely the range of compound and reactions that were discussed.

The year in which his 1952 paper was published was the same as that of Professor Mulliken‘s publication of the important paper on the chargetransfer force in donor-acceptor complexes. Influenced by this paper, he gave a theoretical foundation for the findings mentioned above.
The basic idea was essentially the consideration of the importance of the electron delocalization between the frontier orbitals of reactant species. The frontier orbital approach was further developed in various directions by his own group and many other scientists, both theoretical and experimental.
He was awarded the Nobel Prize in Chemistry 1981. In chemical reactions, molecules composed of atoms meet and form new compounds. Electrons orbiting around the atoms' nuclei play an important role here.
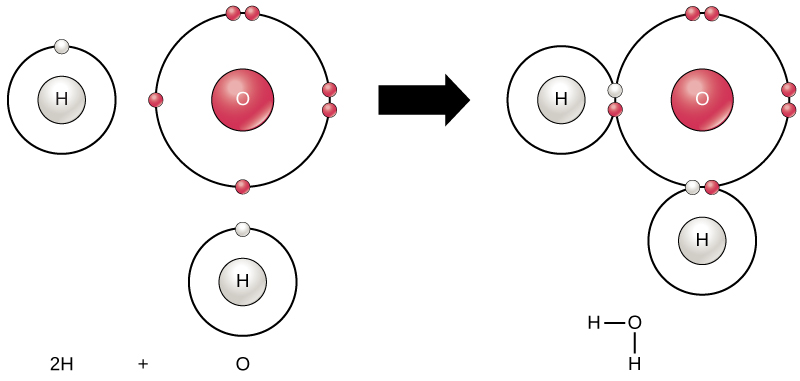
In 1952, Kenichi Fukui developed a theory that showed that the properties of the orbits of electrons that are most weakly bonded to the atom are critically important in understanding chemical reactions. In later, more developed theories, Kenichi Fukui and Roald Hoffmann proved independently of one another how the symmetrical properties of electron orbitals explain the course of chemical reactions.
According to nobelprize











